Sep 20, 2021 · 6 min read
5 Scientists Pushing the Boundaries of Imaging Technology
In the dynamic field of visual proteomics, scientists are developing cutting-edge technologies to study our cells at near-atomic resolution. Their efforts will help us learn more about the functions of the millions of proteins inside each of our trillions of cells — allowing us to better understand and treat disease.
Earlier this year, CZI announced $28 million in funding for 14 projects to advance technology developments in visual proteomics. The teams will work on technology projects that focus on everything from improvements to imaging hardware to new software development to imaging probes. Their work is part of a broader initiative, called the Frontiers of Imaging, designed to accelerate the next generation of advances in biomedical imaging.
I have almost 30 years of experience in electron microscopy of cellular structures, but the field has never been more exciting than right now.
Meet some of the scientists leading projects for the visual proteomics grant and learn more about how they are driving technical advances in imaging technology.
Note: Some of the excerpts below were condensed and edited for clarity.
Justin Taraska, PhD, National Institutes of Health

The cell membrane is key to all cellular life: it’s the protective boundary around our cells that controls what goes out and comes in. It also plays a major role in helping cells communicate with one another and the outside world. Justin Taraska is collaborating with a cross-institute team of three labs — including Jenny Hinshaw, Naoko Mizuno, and Kem Sochacki — to develop tools to map the proteins and structures on the inner and outer surfaces of cells.
“We hope to understand how the plasma membrane allows cells to communicate and interact with other cells and the outside world,” explains Justin.
Daniela Nicastro, PhD, UT Southwestern Medical Center

Daniela Nicastro and her team members use a powerful technique called cryo-electron tomography (cryo-ET) to view the inner workings of cells at molecular or near-atomic resolution. While this technique helps researchers view cells in stunning detail, it’s not compatible with the standard fluorescent labeling techniques researchers typically use to pick apart different proteins and structures within cells by light microscopy.
To help researchers better understand what they’re capturing, the Nicastro lab is working to design new ways of labeling molecules for cryo-ET that will help “color” the details and may revolutionize the field. The technological development proposed here has the potential to transform structural proteomics and the field of cell biology.
Says Daniela of her work, “I have almost 30 years of experience in electron microscopy of cellular structures, but the field has never been more exciting than right now.”
Julia Mahamid, PhD, European Molecular Biology Laboratory

State-of-the-art microscopes let us see more details in a cell, but the more detail, the more data there is to comb through to find what you’re looking for. Julia Mahamid, along with a team of researchers, is harnessing the power of combining cryo-superresolution light microscopy and electron tomography through machine learning to more easily find and capture the details of small and less abundant parts of the cell, starting in bacteria then adapting their approach to more complex organisms.
“With our imaging approach, we want to get closer to understanding how the combined action of different molecular machines ultimately leads to life,” says Julia.
Johann Danzl, PhD, MD, Institute of Science and Technology, Austria
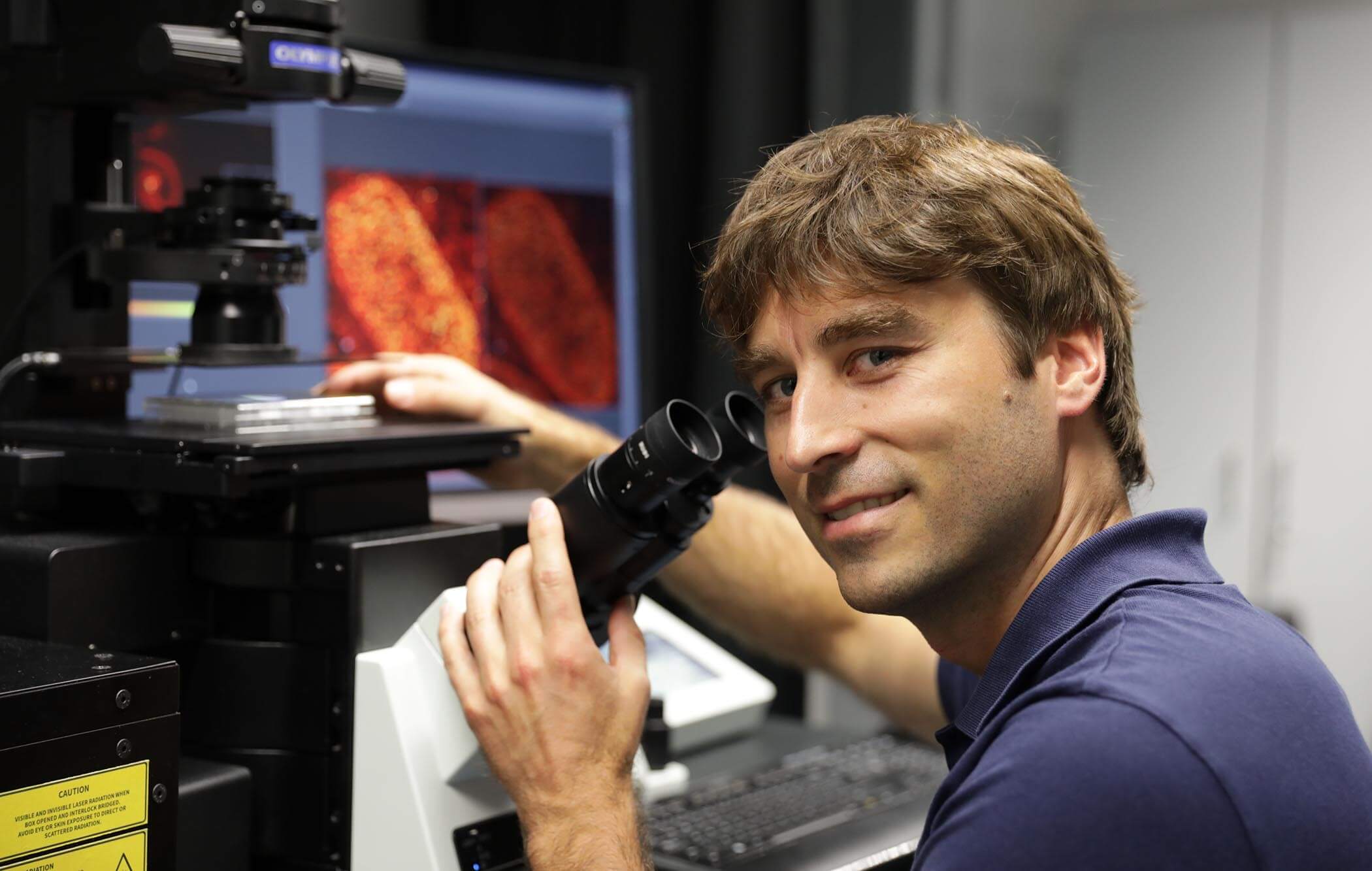
If you think finding a needle in a haystack is hard, imagine trying to find a specific, tiny protein amongst the millions floating in the cytoplasm of a cell. Now imagine the structure of that protein is previously unknown! Johann Georg Danzl, along with a team of physicists and structural cell biologists, is working to make this a less arduous task by building tools, including a novel cryo-superresolution microscope, to reveal the location of proteins at nanometer resolution and uncover unknown structures.
They are adapting super-resolution microscopy techniques for cryo-imaging and bringing them to the nanometer scale so they can better take a census of the many proteins in a cell. Their approach will help reduce the noise of a crowded cell so researchers can more easily determine structures of different proteins of interest directly within cells and gain a clearer picture of the molecular architecture of our cells.
On what drives his interest in the imaging field, Johann says, “I find it very rewarding to be inspired by specific biological problems and unmet needs in biological measurement technology.”
Wah Chiu, PhD, Stanford University
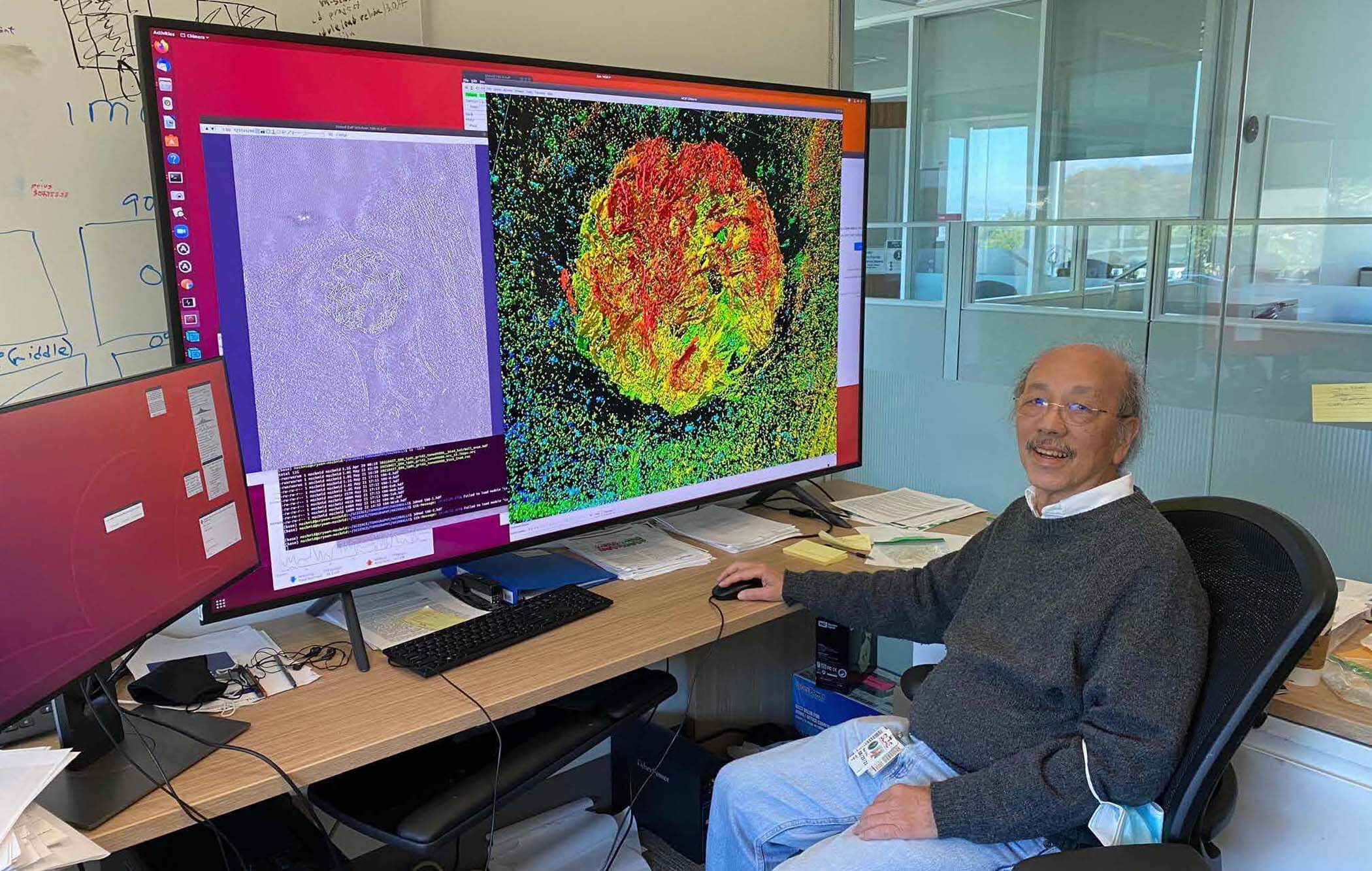
Just like your body has bones that keep you upright, the cell has a cellular skeleton that helps keep its shape and traffic important molecules all around.
Wah Chiu of Stanford University is probing a major skeletal protein of the cell at unprecedented resolution. Wah’s team is taking a hybrid approach to microscopy, combining fluorescent labeling techniques that help identify unique structures and cryo-electron microscopy techniques that magnify cells at high resolution. By doing this, they’re creating an adaptable pipeline that will help researchers create a comprehensive atlas of the proteins that make up the structure of any cell.
“These can lead to clues to develop drugs or vaccines against cells when they cause human diseases,” explains Wah.
To learn more about the CZI grantees leading technology advances in visual proteomics, view the full list of projects.