Nov 1, 2024 · 5 min read
How Biohub Collaboration Helped Unravel a Medical Mystery
At our Biohubs, researchers from different fields are coming together to tackle complex scientific challenges. Through collaboration and cutting-edge science, we’re building solutions for the long term.

During the early days of the pandemic, doctors were struggling to understand why a small fraction of kids who’d been infected with the COVID virus — and successfully fought it off — started suffering from severe inflammation and organ failure weeks later.
The condition, called MIS-C, has life-threatening consequences. The cause was a mystery until researchers made a groundbreaking discovery earlier this year. It turns out kids with MIS-C were producing antibodies and T cells that targeted not only the COVID virus, but also their own immune system.
There’s a fascinating story behind that discovery, and one of the keys to unlocking this mystery came from our San Francisco Biohub president, Joe DeRisi. He and a team of scientists had been using a tool to study antibodies called phage immunoprecipitation sequencing (PhIP-Seq). Scientists at UCSF and the San Francisco Biohub, in partnership with doctors at St. Jude Children’s Research Hospital and Boston Children’s Hospital, used PhIP-Seq to discover the root causes of MIS-C. This discovery not only unraveled the MIS-C puzzle, but has now opened new avenues for understanding autoimmune diseases more broadly.
Expanding What’s Possible in Bioengineering and Immune Research
In many ways, this story embodies what we’re trying to do at the San Francisco Biohub and across the Biohub Network. Our goal is to give brilliant researchers the opportunity to take on hard problems in science; to partner across disciplines and institutions; to develop powerful tools and techniques; and ultimately, to make discoveries that couldn’t happen in any other setting.
Sometimes those discoveries pertain to a specific disease. But more often, we’re trying to understand the most fundamental parts of human biology — the basic mechanisms of how healthy cells function — and what gets disrupted during states of disease. Our network, which began in San Francisco in 2016, now includes research institutes doing cutting-edge science in Chicago, New York and Redwood City, California.
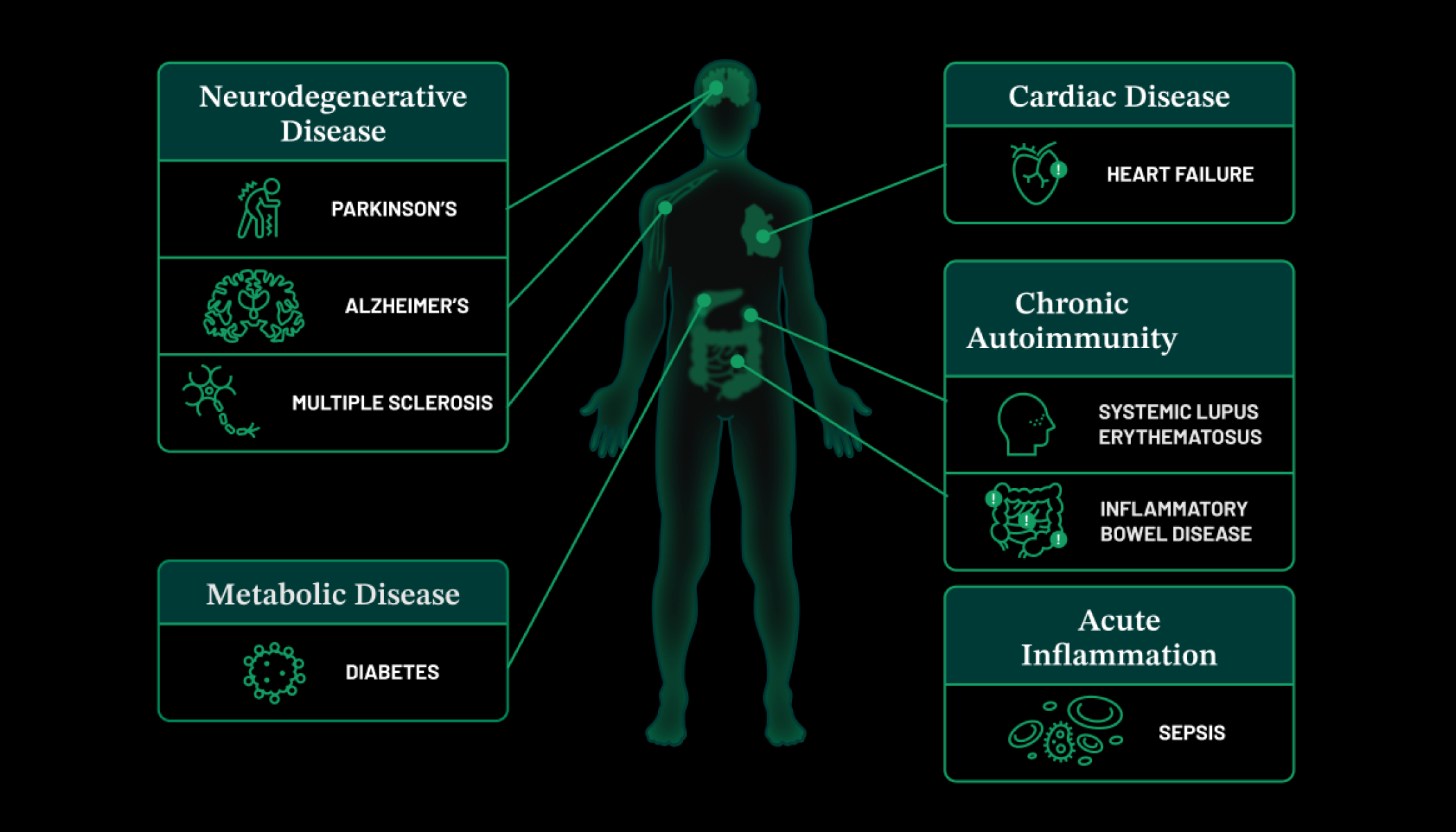
At the Chicago Biohub, for example, researchers are engineering tissues and developing microsensors with the goal of understanding inflammation and the immune system. Inflammation and overactive immune cells play a key role in many diseases, including cancer, heart disease and Alzheimer’s. One of the fascinating research projects underway is led by the Microtissue Signaling Dynamics group, which is investigating how signaling networks across tissues and within individual cells interact with each other to coordinate immune response. The team aims to collect data from tissues to create intuitive models that reveal how diseases develop and how cancer forms. The ultimate goal is to use these insights to develop therapeutic strategies for treating autoimmune diseases and cancer.
Meanwhile, researchers at the New York Biohub have begun their work to bioengineer new capabilities into human immune cells that can help detect, prevent, and eventually treat diseases, such as ovarian and pancreatic cancers, and neurodegenerative diseases, at the earliest possible opportunity. For example, researcher Gordana Vunjak-Novaković is exploring how cancer cells can avoid detection by the immune system. (And how we might one day be able to fix that.) She’s developing “organ-on-a-chip” tools — small, bioengineered devices that mimic human organs and can teach us a lot about how tumors use chemical signals to evade the body’s defense mechanisms.
Seeing Really Tiny Things With Powerful Microscopes
Another way to study how cells behave is by looking at them directly. I recently paid a visit to our Imaging Institute, where teams of scientists and engineers are using some of the world’s most powerful microscopes to image cells in unprecedented detail — not as static objects, but as systems in motion.
These images, called tomograms, are critical windows into cellular health and disease. However, pulling data from tomograms that can be used by scientists to drive discovery is a tedious, time-consuming, and often manual process. To help solve this bottleneck, the Imaging Institute has created the CryoET Data Portal, an open-access portal that shares high-quality tomogram data so that other scientists and machine learning experts can develop better, faster annotation models. There are now over 15,000 tomograms in the CryoET Data Portal for analysis and model training, and we hope to continue to expand the CryoET Data Portal so researchers can better understand how healthy cells work, and the changes associated with disease.
Biohub Research + AI = the Future of Science
One of our big bets at CZI is that by training AI on all of this cellular data — the imaging data from our Imaging Institute, the molecular sequencing data from the Biohubs, and other sources from across the scientific ecosystem — we can create virtual models of human cells.
I joined our head of science, Steve Quake, at Stanford’s Big Ideas in Medicine event to discuss what virtual cell models might mean for the future of science. Looking into the future, some of the potential applications we’re most excited about: If you were studying a genetic disease, you could plug in a mutation into the model and simulate how it affects a particular cell type. You could identify the proteins and processes that get disrupted. You could test a myriad of possible drug candidates. And you could screen those drugs for side effects.
More on how we’re working with AI: Our Progress Leveraging AI To Speed Up Biomedical Research
Looking Ahead
This kind of science — where researchers can leverage advances in AI to unlock new understandings of cells and cell systems — has the potential to radically accelerate the pace of scientific breakthroughs. The intersection of AI, advanced imaging and collaborative research is opening up possibilities that were unimaginable just a few years ago. And, as the MIS-C story reveals, we’re doing it all while building a culture that prioritizes collective discovery over individual recognition, supported by open access to advanced tools and diverse expertise.
With care,
Co-Founder and Co-CEO
Chan Zuckerberg Initiative