Oct 29, 2020 · 8 min read · 1 photos
How Breakthroughs in the Science of Inflammation Could Lead to Healthier Pregnancies

It’s often said that the human brain is the most complex object in the known universe. But the immune system is, at the very least, a close runner-up.
For one thing, the immune system comprises an extraordinarily diverse network of cells. There are cells to destroy infected cells, and cells to produce antibodies. Others act like a thermostat for your immune system, dialing it up or down as necessary. There are also proteins called immunoglobulins, which are tailor-made to neutralize any of the millions of pathogens that can enter the body. And there are undoubtedly many immune cell types that haven’t been discovered yet, with properties and functions that we can only imagine.
All of these cells are in constant conversation with other specialized cell types in our body. Together, they form a dynamic system that can not only repel infections from the day you’re born, but learn over time—adapting to fight new pathogens, and creating a kind of lifelong immune memory.
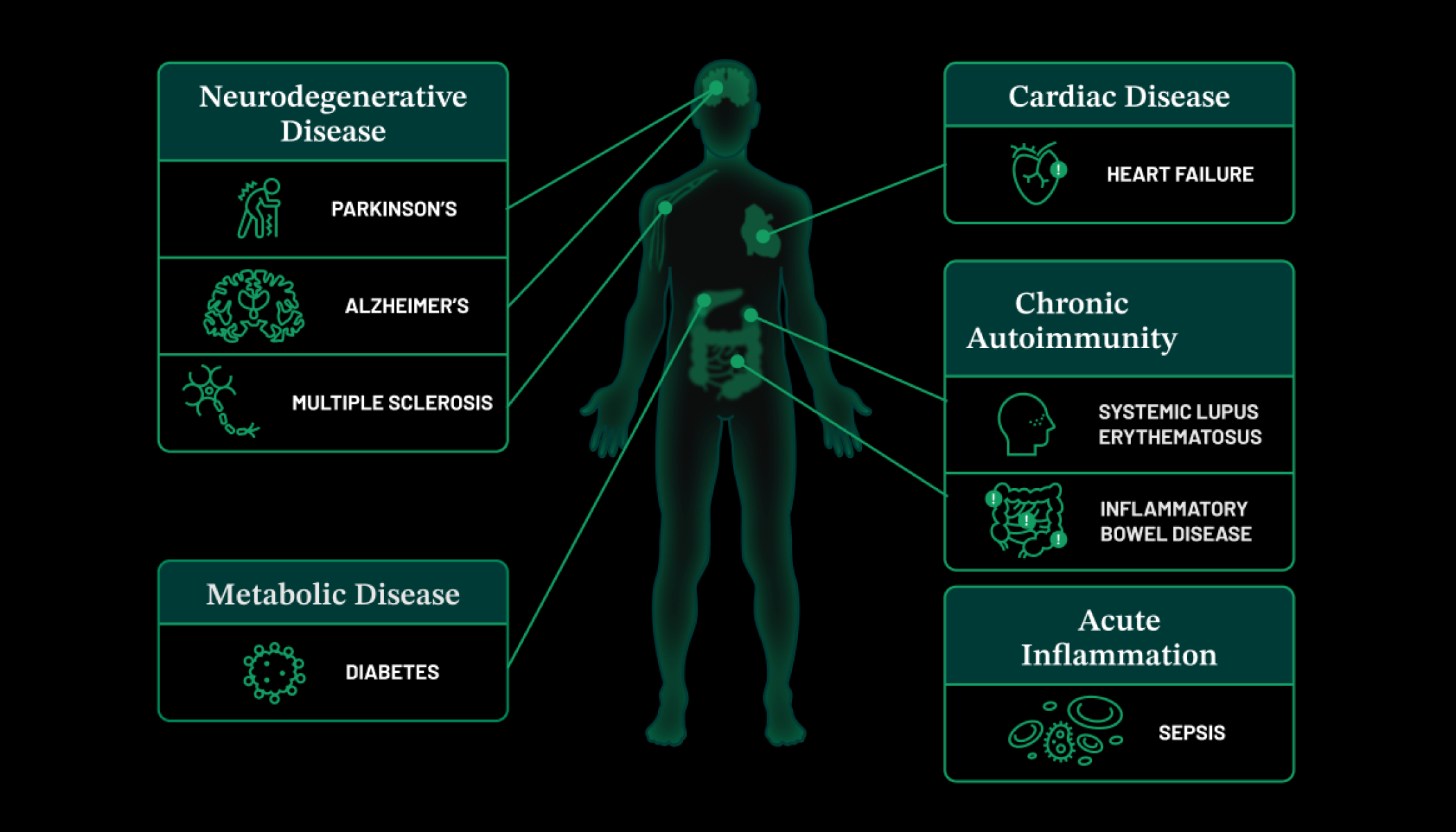
Scientists are only beginning to understand how this system works. We know, for example, that chronic inflammation—a long-term, ongoing immune response—is a cause or a consequence of many diseases, including cancer, heart disease, diabetes, and Alzheimer’s. But the exact roles that inflammation plays are unclear. This is especially true of chronic inflammation in pregnant women, which can affect how a baby develops—and in some cases, may even manifest as a disease later in that person’s life.
With so much at stake, it’s clear we need a far better understanding of how the immune system functions than we have now. The good news is, we’re poised for a breakthrough.
The promise of single-cell biology
One of the reasons we don’t know a lot about inflammation is that we’ve had trouble getting a good look at the immune cells that cause it. Historically, when researchers sampled an inflamed tissue, they had to examine thousands or millions of cells at a time. The odds of finding and characterizing a rare, but potentially important cell type—of which there are many in the immune system—were low. And our ability to see how those cells interact was even worse.
Then, about ten years ago, things started to change. Biologists began using a set of techniques that allowed them to probe deeply into individual cells—a discipline that came to be known as single-cell biology.
One of the foundational insights behind single-cell biology is that we can look at the way a cell expresses its genome to learn what it does. Every cell in your body has more or less the same DNA. However, the way that DNA gets “expressed”—the sections that get “turned on,” and ultimately translated into proteins—vary radically between different cell types. By measuring molecules that handle the translation, called RNA, scientists can glean a lot about what a cell is made of and how it functions.
Recent advances in technology have made it possible to apply this technique at an important level of resolution that was previously not feasible: the individual cell. And researchers are using it to map the human body—including the immune system—in a way that was never possible before.
A network of individual cells
Over the last several years, CZI has made a big commitment to funding single-cell biology research. One of the global projects we support, called the Human Cell Atlas, aims to create a reference map of every cell type in the human body. No such resource has ever existed; if it did, it would dramatically enhance our understanding of health and disease. Early progress among researchers working to compile the Human Cell Atlas have demonstrated the power of this resource by helping to clarify diverse diseases including COVID-19, cystic fibrosis and others.
Another set of CZI supported projects looks specifically at the immune system and inflammation. To date, most immunology research has focused on specific diseases and specific tissues. But a new cohort of researchers are using single-cell biology to see how networks of immune cells coordinate and communicate across the body—and what that might reveal about the immune system as a whole.
CZI made grants to 29 of these research teams earlier this year. A few even proposed to study the immune response to coronaviruses before the current pandemic. Others are imaging immune cells in the gut. And two are looking at how inflammation can affect a pregnancy—an area of research that is often underfunded.
Revealing the role of inflammation in pregnancy
One of those researchers is Dr. Kellie Ann Jurado, an immunobiologist at the University of Pennsylvania. She and her colleagues, Dr. Dan Dongeun Huh and Dr. Monica Mainigi, will investigate how a pregnant woman’s immune system interacts with the fetal immune system through the placenta—and the ways maternal inflammation can disrupt the delicate balance that’s needed for a successful pregnancy.
“We know inflammation can have negative consequences during pregnancy,” Dr. Jurado says, “but it also serves an essential role.” Too much can lead to premature birth, preeclampsia, and other health risks. Without some immune protection, though, a child would be vulnerable.
Using techniques from single-cell biology, Dr. Jurado and her team hope to discover the molecular basis for these observations: the genes that are active in maternal and fetal immune cells, the cell types they’re found in, and how these cells interact as a network.
Dr. Jurado is also paying particular attention to the role inflammation plays in establishing a pregnancy. “Our findings have the potential to guide the use of immunomodulators, which can help us prevent or treat early pregnancy complications and infertility,” she says.
Finding the hidden link between stress and pregnancy outcomes

“From daily life, we know we’re all struggling with a lot of stress,” Dr. Li says. “So my whole experiment is designed to understand how this stress could be translated into biological signals, leading to inflammatory responses and adverse pregnancy outcomes.”
The research takes on even greater relevance in light of the fact that in the U.S., on average, Black women experience greater amounts of chronic stress and higher rates of chronic inflammation—factors that may contribute to the three-year life expectancy gap between Black and white women.
Black women also experience worse pregnancy outcomes on average, and are three to four times more likely to die during childbirth than white mothers.
Dr. Li aims to draw out the biological mechanisms that could explain these disparities, and eventually lead to effective interventions and therapies. He is joined by Dr. Gary Shaw and Dr. David K. Stevenson of Stanford Medicine’s Department of Pediatrics, who will combine their expertise in genomics, epidemiology, and clinical analysis to address a highly interdisciplinary problem.
Like Dr. Jurado, Dr. Li and his colleagues will use single-cell techniques to learn more about immune cells in the placenta. He and his team will see which cell types are especially receptive to the biological markers of stress, and when and how they trigger an inflammatory response.
A new research network—and a new understanding of the immune system
While Dr. Jurado and Dr. Li are using different techniques to explore different questions, we at CZI hope that their research—combined with that of other grantees—could help us see the immune system in an entirely new light.
“What we’re really trying to do is build a network of investigators that can find emergent trends, themes, cells and pathways in the immune system,” says Jonah Cool, the lead for CZI’s Single-Cell Biology Program. “We hope to clarify emergent properties of the inflammation process that could tell us about many diseases.”
In the near term, those insights could help us take better care of ourselves. For example, if you’re pregnant, you might not simply be told that stress and inflammation are bad, and that you should try to avoid them. You would actually learn about your particular risk factors, and how you could manage them over the course of a pregnancy.
Further out, this kind of research could pinpoint which cells perform what functions within the immune system, which could ultimately help us target existing drugs more effectively, or develop new ones. One day, your doctor might even be able introduce or deplete certain cell types to help you fight a disease.
In partnership with pioneering scientists like Dr. Li and Dr. Jurado, CZI will keep working toward that future.